Recently, Professor Ji Chunyan’s team from Department of Hematology, Qilu Hospital of Shandong University, has made great progress in the field of tumor immune microenvironment remodeling and immunotherapy for primary central nervous system lymphoma (PCNSL). The related article "Spatial single cell analysis of tumor microenvironment remodeling pattern in primary central nervous system lymphoma" was published online in the prestigious international journal
Leukemia (CAS District 1, IF=12.883). Based on the first paraffin section space transcriptome detection platform in China, and relying on the team's own bioinformatics analysis team, this study systematically revealed the spatial characteristics and immune remodeling mechanism of PCNSL tumor microenvironment for the first time. Professor Ji Chunyan and Professor Ye Jingjing of Hematology Department of Qilu Hospital of Shandong University are co-corresponding authors, Dr. Xia Yuan and Professor Sun Tao are co-first authors. Qilu Hospital of Shandong University is the first author and communication author’s affiliation.
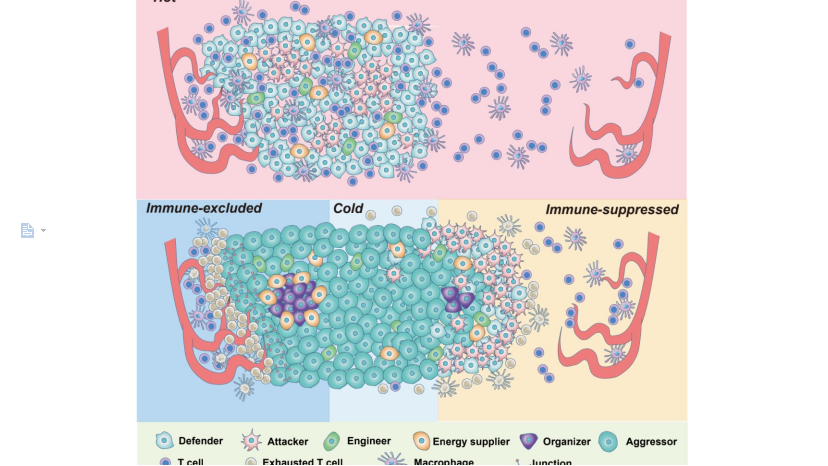
PCNSL is a rare extranodal non-Hodgkin's lymphoma that occurs mostly in the skull, spinal cord, meninges, and eyes. It is highly aggressive, and has an extremely poor prognosis for patients who fail first-line therapy. The tumor microenvironment (TME) plays an important role in tumor’s genesis, progression, metastasis, and drug sensitivity. Based on the interaction mode between tumor and immune system, TME falls into 4 categories, "Hot", "Immune rejection (IME)", "Immunosuppressive (IMS)" and "Cold". At present, most studies still focus on the analysis of the cellular composition of TMEs, and it is of great significance for the development of immunotherapy strategies to clarify the spatial distribution characteristics of multiple cells in different TMEs and the spatial remodeling mechanisms of different TMEs during tumor development.
This study combined spatial transcriptomic data of four typical TME samples of PCNSL with 14,964 single-cell transcriptomic data, and defined a new subpopulation of PCNSL tumor cells with spatial functional characteristics through integrated analysis. Subsequently, developmental trajectory analysis revealed the developmental order of four TME types during PCNSL progression, and confirmed that the tumor cell subpopulation could remodel TME by "immune stress-sensing model". After elucidating the specific mechanism and key molecules of the "immune stress-sensing model" through spatial communication analysis, the study further explored the spatial and temporal distribution and dynamic changes of typical immune checkpoint molecules and CAR-T target molecules in PCNSL, providing a basis for precise immunotherapy of PCNSL. The study also provides an important reference for the elucidation of TME remodeling mechanisms in other tumors.
Professor Ji Chunyan's team has been committed to basic and clinical research on hematological tumors for a long time, devoting to the innovation and clinical transformation of basic research. At the same time, his team actively promote the interdisciplinary integration, and try to make breakthrough in accurate diagnosis and treatment technology of hematological tumors. In recent years, Prof. Ji’s team has made many innovative achievements in the pathogenesis and drug resistance mechanism of hematological tumors, targeted therapy and accurate diagnosis and treatment of artificial intelligence, which have been funded by National Natural Science Foundation (including major research projects), Mount Tai Scholars Climbing Program and many provincial and ministerial programs. The team has published more than 100 articles in high-level academic journals, including
Advanced Science, Journal of Hematology & Oncology, and
Leukemia. The main research results "Abnormal Biological Behavior of Leukemia and its Reversal Countermeasures" and "R&D, Popularization and Application of Key Technologies for Accurate Diagnosis and Treatment of Leukemia" won the first prize of the Shandong Provincial Scientific and Technological Progress Prize in 2012 and 2021 respectively.
Link:
https://www.nature.com/articles/s41375-023-01908-x
